Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic, autoimmune disorder in which the body’s immune system attacks healthy cells. The disease is characterized by the presence of autoantibodies in the blood, and is an antigen-driven autoimmune disease. Symptoms can range from mild to severe, and may be accompanied by other comorbid conditions.
Antigen-driven autoimmune disease
rheumatoid arthritis (RA) is a chronic inflammatory disease that is characterized by progressive damage to joints. The main mechanism for destruction is local induction of metalloproteases, which leads to the destruction of cartilage, bones, and other tissues. During the inflammatory process, GM-CSF is secreted by pro-inflammatory PD1highCD8+ T cells. This results in tissue damage, with a focus on extra-muscle tissues such as tendons, ligaments, and vascular structures.
Ectopic lymphoid neogenesis is the formation of follicular dendritic cell networks in autoimmune conditions. These structures are characterized by the aggregation of T and B cells. They develop at sites of inflammation and infection in target tissues and may form in tumors, gut-associated lymphoid tissue, and graft rejection.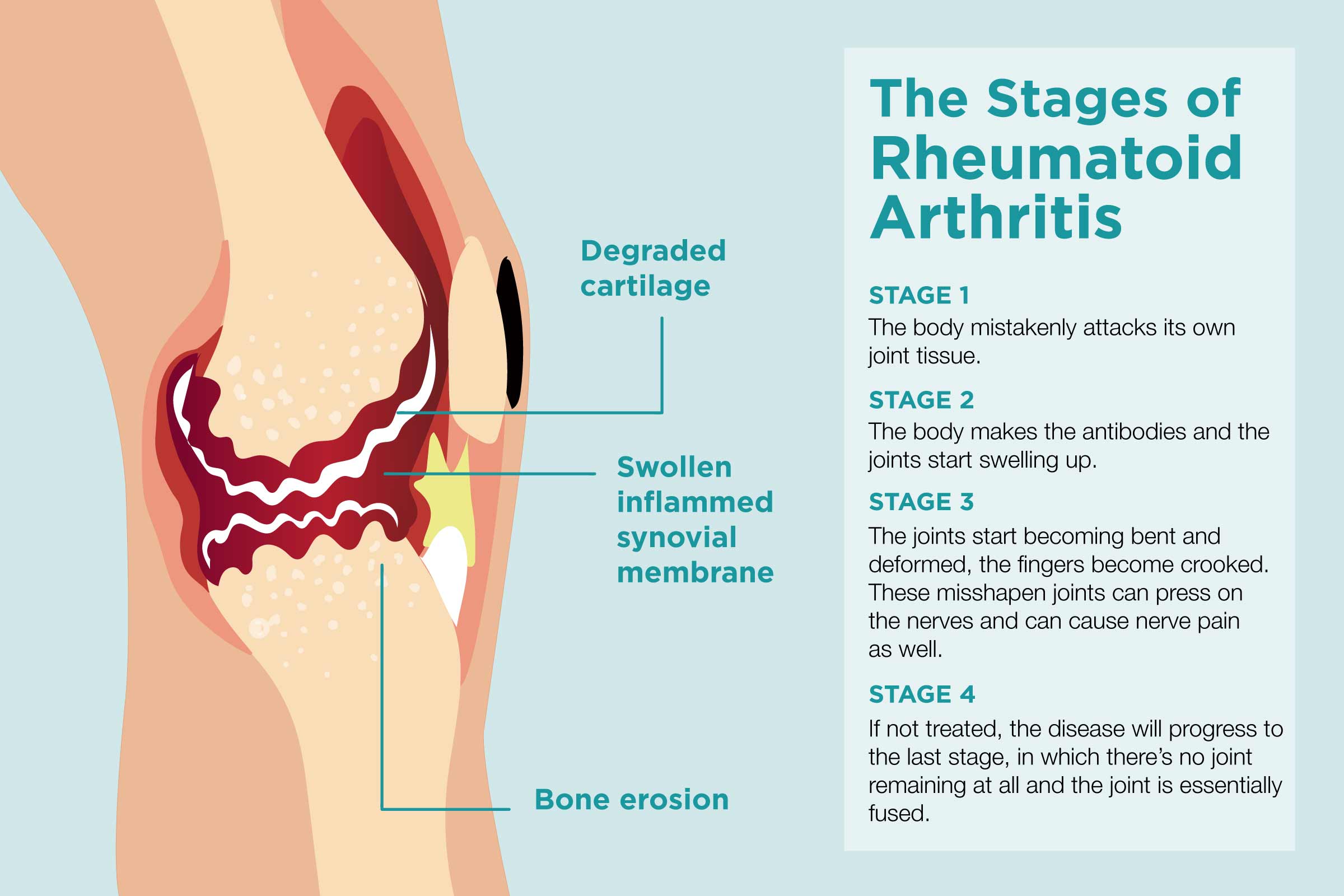
The development of these structures is a major feature of chronic autoimmune diseases and is found in many autoimmune disorders. However, the underlying processes are not understood. Identifying the antigens that drive both B and T cell responses could lead to future vaccines, and identify novel targets for treatment.
Serum IgMs are predominantly B1 B-cell-derived natural autoantibodies
B cells have a critical role in regulating adaptive immunity, including the production of antibodies. These cells have been shown to contribute to the development of naive CD4+ T cell responses and to be involved in complement fixation and opsonization. Moreover, a subpopulation of B cells that promotes macrophage activation has been identified. However, the nonclassical immune functions of B cells have not been explored.
In the current study, a specific subset of B cells, called CD11ahiFcgRIIIhi B cells, were identified and characterized. The expression of their transcription factors and co-stimulation molecules was evaluated, and their transcriptional signature was compared with that of conventional B cells. This analysis revealed that the CD11ahiFcgRIIIhi subset expresses a unique phenotype and has a reduced expression of MHC-II. They secrete significantly lower levels of IgG3 and IgM than conventional B cells.
In addition to producing IgG3, these B cells are able to produce IgM, an autoantibody. Because of this ability, these cells are considered to be important contributors to the humoral immune response.
IL-23 could be a useful marker for disease activity in RA
In a recent study, IL-23 level has been shown to be a good indicator of active disease in RA patients. These findings are in accordance with the findings of other studies that have investigated the disease activity of RA patients.
IL-23 is produced by cells that secrete other pro-inflammatory cytokines. It is a key cytokine controlling inflammation in peripheral tissues. Several autoimmune diseases are associated with chronic inflammation. RA is a chronic inflammatory autoimmune systemic disorder characterized by articular cartilage destruction and joint swelling.
Several studies have reported that serum levels of IL-23 are significantly higher in RA patients than in healthy control groups. This finding may be of importance in identifying patients with active RA, especially as they continue to deteriorate.
Studies have also shown that the protein leucine-rich a2 (LRG) is an important marker of active inflammatory processes. LRG is a glycoprotein that is upregulated during the active phase of inflammatory processes and is induced by other pro-inflammatory cytokines such as IL-6. The expression of LRG has been observed in macrophage lineage cells in RA patients.
Comorbidities
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease, often accompanied by several comorbid conditions. These conditions can impact both the severity and quality of life of patients. A comprehensive approach to RA requires adequate recognition of these comorbidities and the appropriate management of them.
Comorbidities in RA are associated with an increased risk of treatment-related complications and increased mortality. However, the relationship between inflammation and the occurrence of these comorbidities is not well understood. Several studies have focused on assessing the presence of comorbidities in RA patients.